The transition from traditional lithium-ion batteries to solid state batteries represents one of the most significant technological shifts in modern energy storage. These batteries promise higher safety, longer lifespan, and greater energy density — all while addressing the key limitations of current lithium-ion technology. As the industry evolves, hybrid innovations like the semi-solid state battery are also gaining attention for bridging the gap between conventional and fully solid-state solutions. But understanding how a solid state battery actually works requires exploring its materials, chemistry, and the fundamental difference between liquid and solid electrolytes.
The Basic Structure of a Solid State Battery
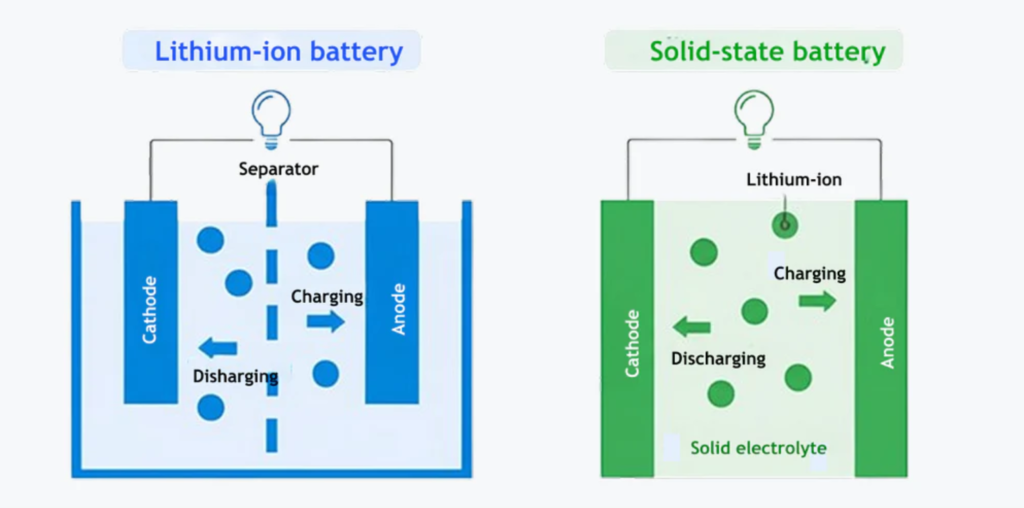
A solid state battery functions on the same fundamental principle as other rechargeable batteries — by moving ions between a cathode and an anode through an electrolyte. However, the main difference lies in the type of electrolyte used.
In conventional lithium-ion batteries, the electrolyte is a liquid or gel that allows lithium ions to move during charge and discharge cycles. In a solid state battery, this liquid is replaced with a solid electrolyte. This change may seem simple, but it transforms the battery’s behavior, stability, and energy potential. Today, even transitional technologies like the semi-solid state battery are being explored to enhance safety and performance while maintaining some of the manufacturing advantages of liquid systems.
| Feature | Liquid Electrolyte (Lithium-Ion) | Solid Electrolyte (Solid State) |
| Material | Liquid or gel-based lithium salts | Ceramic, glass, or polymer solids |
| Safety | Risk of leakage and fire | High thermal and chemical stability |
| Energy Density | Moderate | Significantly higher |
| Lifespan | Shorter cycle life | Longer cycle life |
Key Components of Solid State Batteries
A solid state battery consists of three main components — the cathode, anode, and solid electrolyte. Each plays a distinct role in ensuring smooth ionic movement and energy transfer.
- Cathode: Usually made from lithium-based compounds such as lithium cobalt oxide (LiCoO₂) or lithium iron phosphate (LiFePO₄ battery) materials. The cathode stores lithium ions during discharge.
- Anode: Often composed of lithium metal or graphite. In solid state designs, pure lithium metal is preferred because it offers higher energy density.
- Solid Electrolyte: This replaces the liquid electrolyte found in conventional batteries. It acts as a separator and ionic conductor while remaining electronically insulating.
The combination of these components allows the battery to transfer energy more efficiently and safely compared to traditional designs.
How Energy Transfer Works in Solid State Batteries
The process of charging and discharging in a solid state battery follows the same electrochemical principles as in conventional batteries, but with different physical behaviors.
- During Charging: Lithium ions move from the cathode, pass through the solid electrolyte, and are stored in the anode as lithium metal.
- During Discharging: The ions travel back to the cathode, releasing stored energy to power external devices.
Because the electrolyte is solid, the movement of ions is highly structured and stable. This improves the overall lifespan and minimizes risks like short-circuiting or thermal runaway.
The Role of Solid Electrolytes
The solid electrolyte is the heart of a solid state battery. It determines the battery’s performance, efficiency, and operational safety. The electrolyte must have high ionic conductivity, stability against both electrodes, and good mechanical strength.
Common materials used as solid electrolytes include:
- Ceramic Electrolytes: Known for high ionic conductivity and excellent stability.
- Polymer Electrolytes: Flexible and lightweight, often used in portable applications.
- Sulfide Electrolytes: Provide excellent ionic movement, ideal for high-energy batteries.
Each type has its own advantages and challenges, which determine how the battery performs in real-world conditions.
Comparison: Solid State Battery vs Lithium Ion
To understand the true impact of this technology, it’s important to compare a solid state battery vs lithium ion battery directly. While both rely on lithium ions for charge transfer, the replacement of liquid with solid materials creates major differences in performance and durability.
Solid State Battery vs Lithium Ion Battery
| Aspect | Lithium-Ion Battery | Solid State Battery |
| Electrolyte | Liquid or gel | Solid ceramic or polymer |
| Safety | Flammable, risk of leakage | Non-flammable and stable |
| Energy Density | 150–250 Wh/kg | 300–500 Wh/kg (potentially) |
| Operating Temperature | 0°C to 60°C | -20°C to 100°C |
| Cycle Life | 500–1500 cycles | 2000+ cycles |
| Cost | Lower currently | Higher but falling with scale |
This comparison highlights why industries such as electric vehicles (EVs), aerospace, and grid storage are actively investing in solid state battery research.
Advantages of Solid State Batteries
- Enhanced Safety: The solid electrolyte eliminates the risk of leakage and combustion, making the battery far safer than its liquid-based counterparts.
- Higher Energy Density: More lithium can be stored in the anode, allowing longer operation times in smaller battery packs.
- Longer Lifespan: Reduced degradation means a solid state battery can last twice or even three times longer than a lithium-ion battery.
- Faster Charging: Solid electrolytes enable quicker ion transport, resulting in shorter charging times.
- Wide Temperature Range: These batteries perform well in both hot and cold environments, expanding their usability.
These advantages explain why many global manufacturers are moving toward solid state technology as the future of energy storage.
Challenges and Limitations
Despite their benefits, solid state batteries face several technical challenges that have slowed mass commercialization.
- Manufacturing Complexity: The fabrication of solid electrolytes requires precise control over materials and temperatures.
- Interface Resistance: Ensuring seamless contact between solid layers is difficult and can affect ionic flow.
- Cost: The materials used, such as ceramics and sulfides, are expensive compared to liquid electrolytes.
- Scalability: Producing large-scale solid state cells consistently remains a challenge for manufacturers.
These barriers are actively being addressed through material innovations, such as semi-solid state battery designs that combine liquid and solid properties.
Semi-Solid State Battery: A Transitional Technology
A semi-solid state battery serves as a bridge between conventional lithium-ion and fully solid state designs. It combines aspects of both — using a thick gel or partially solid electrolyte to enhance stability while maintaining efficient ion transfer.
In this structure, the electrolyte is not completely solid but more viscous than traditional liquids. This design offers improved safety and energy density while being easier to manufacture.
Benefits of Semi-Solid State Batteries:
- Lower risk of short circuits compared to liquid systems.
- Easier production process than full solid state cells.
- Enhanced durability and better thermal management.
The semi-solid state battery is seen as a practical step toward fully solid technologies that can be mass-produced for electric vehicles and renewable energy storage.
LiFePO4 Battery and Its Relationship to Solid State Design
Another important variant in lithium technology is the LiFePO4 battery (Lithium Iron Phosphate). It is not a solid state battery, but its chemistry offers excellent thermal stability and safety, similar to solid state characteristics.
LiFePO4 batteries are often used in solar energy systems, electric vehicles, and backup power because they are:
- More stable under high temperatures.
- Resistant to overcharging.
- Capable of long cycle life (2000+ cycles).
Researchers are exploring how solid state electrolytes can be paired with LiFePO4 cathodes to further enhance their efficiency. This hybrid approach could result in safer, high-performance energy systems with minimal degradation.
How Solid State Batteries Are Made
The manufacturing process for a solid state battery differs greatly from that of lithium-ion cells. Instead of stacking liquid components, solid materials are compressed into thin layers under high pressure.
The process includes:
- Material Preparation: Lithium metal, cathode powder, and electrolyte materials are prepared under controlled conditions.
- Layer Assembly: The cathode, electrolyte, and anode are pressed together to form a dense, multi-layered structure.
- Sintering or Coating: High-temperature sintering helps bind the solid electrolyte to the electrodes.
- Encapsulation: The cell is sealed to prevent contamination and ensure stability during cycling.
This process requires advanced machinery but results in a compact and stable battery with reduced failure rates.
Applications of Solid State Batteries
Solid state batteries are becoming essential in various high-demand sectors:
- Electric Vehicles (EVs): Their higher energy density and safety make them ideal for EV manufacturers looking for longer range and faster charging.
- Consumer Electronics: Smartphones, laptops, and wearables benefit from thin, lightweight batteries.
- Aerospace and Defense: Solid electrolytes are stable in extreme environments, making them suitable for critical missions.
- Renewable Energy Storage: They can efficiently store energy from solar and wind systems with minimal degradation over time.
As production costs fall, solid state technology is expected to dominate these industries in the coming decade.
Future Developments and Innovations
Researchers are continuously developing ways to improve solid state battery performance and reduce production costs. Some of the most promising innovations include:
- Using composite electrolytes that combine ceramic and polymer materials.
- Developing thin-film batteries for portable electronics.
- Integrating lithium metal anodes to maximize capacity.
- Exploring solid-state versions of LiFePO4 chemistry for enhanced safety.
Major companies and research institutions are investing heavily in pilot lines and prototype vehicles equipped with solid state systems. The technology is evolving quickly toward large-scale adoption.
Environmental Impact and Recycling
Because solid state batteries do not contain flammable or toxic liquid electrolytes, they are safer and more environmentally friendly. The materials used — such as lithium, iron, and ceramic compounds — are easier to recycle than those in liquid-based batteries.
Additionally, their extended lifespan reduces waste generation and the demand for frequent replacements. This contributes to a more sustainable and efficient energy ecosystem.
Renogy’s Role in Next-Generation Battery Technology
Renogy, a global leader in solar panels and energy-storage solutions, is closely aligned with the evolution of advanced battery systems such as solid state and semi-solid designs. While the company currently specializes in LiFePO₄-based storage for solar applications, Renogy continues to explore emerging technologies that improve safety, lifespan, and energy efficiency. As solid state chemistry matures, brands like Renogy are expected to integrate these breakthroughs into future off-grid and renewable systems, making high-performance energy storage more accessible worldwide.
Conclusion
The solid state battery marks a pivotal advancement in energy storage technology. By replacing liquid electrolytes with solid materials, it delivers superior safety, performance, and longevity. While challenges like cost and manufacturing complexity remain, innovations such as semi-solid state batteries and LiFePO4 battery integration are paving the way for scalable, sustainable solutions.
As industries move toward electrification and renewable energy, the solid state battery vs lithium ion debate will likely end in favor of the former — a technology capable of powering the next generation of electric vehicles, smart devices, and clean energy systems with unprecedented efficiency.











